
Chemistry, 20.11.2020 17:00 tsimonej12
What is the molarity of an aqueous solution that contains 0.072 g C2H6O2 per gram of solution? The density of the solution is 1.40 g/mL.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:10, yootmytoot
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution. calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 22:00, Porciabeauty6788
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 22.06.2019 23:00, maddyleighanne
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3
You know the right answer?
What is the molarity of an aqueous solution that contains 0.072 g C2H6O2 per gram of solution? The d...
Questions in other subjects:

Mathematics, 11.09.2020 19:01

English, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

Chemistry, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

Arts, 11.09.2020 19:01

History, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

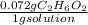 =0.1008 g C₂H₆O₂
=0.1008 g C₂H₆O₂

