
Chemistry, 20.11.2020 14:00 eboniwiley
A piece of iron is heated to 95.0 celsius and then placed in an insulated vessel containing 250. grams of water at 25.0 celsius. when the system comes to equilibrium the temperature of the system is 35.0 celsius. what is the mass in g of the iron? assume no heat is lost to the surroundingsSpecific heat/J. g^-1 K^-1iron 0.450water 4.184 (A) 4.48 g (B) 41.7 g (C) 387 g (D) 612 g

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, kathleendthomas
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1


Chemistry, 21.06.2019 23:30, hellokitty1647
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i. e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1
You know the right answer?
A piece of iron is heated to 95.0 celsius and then placed in an insulated vessel containing 250. gra...
Questions in other subjects:


Mathematics, 09.11.2019 20:31


Advanced Placement (AP), 09.11.2019 20:31



English, 09.11.2019 20:31



Mathematics, 09.11.2019 20:31

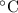 × (308 K - 298 K)
× (308 K - 298 K)

