Look at the balanced equation below:
C2H6O + 3 O2 → 2 CO2 + 3 H2O
If you produced 11.5...


Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, maddynichole2017
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 16:00, matt16913
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 21:20, 50057543
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 23.06.2019 08:00, george27212
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 12.11.2019 14:31




English, 12.11.2019 14:31

English, 12.11.2019 14:31

Mathematics, 12.11.2019 14:31


Spanish, 12.11.2019 14:31

Mathematics, 12.11.2019 14:31

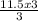 = 11.5moles of oxygen gas
= 11.5moles of oxygen gas

