
Chemistry, 20.11.2020 01:00 shelley3135
Using Models to Answer Questions About Systems
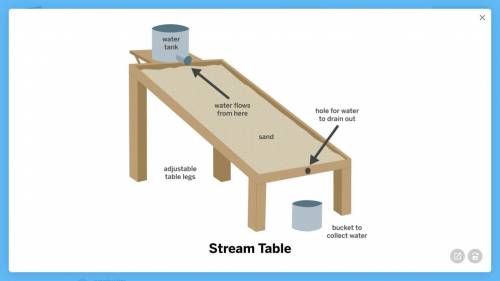
Armando’s class was looking at images of rivers formed by flowing water. Most of the rivers were wide and shallow, but one river was narrow and deep.
Armando’s class thinks that this river is narrow and deep because:
the hill that the water flowed down was very steep, or
the sand grains that the water flowed through were very small.
Armando designed the model below to try to answer the question: Why is this river so narrow and deep?
1a. What features of this model will help Armando answer the question?
1b. How could Armando use this model to test the idea that very steep hills lead to narrow, deep rivers?
1c. How could Armando use this model to test the idea that very small sand grains lead to narrow, deep rivers?
2. Armando thinks that it is the very steep hill that makes this river narrow and deep, but his classmate thinks it is the small size of the sand grains that the water flows through. What results from the model would be evidence that Armando’s idea is correct?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 14:10, cameronbeaugh
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 20:00, teacherpreacher
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
Using Models to Answer Questions About Systems
Armando’s class was looking at images of rivers form...
Questions in other subjects:

Mathematics, 23.12.2020 17:10

Biology, 23.12.2020 17:10




Social Studies, 23.12.2020 17:10






