Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 22:30, teagan56
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
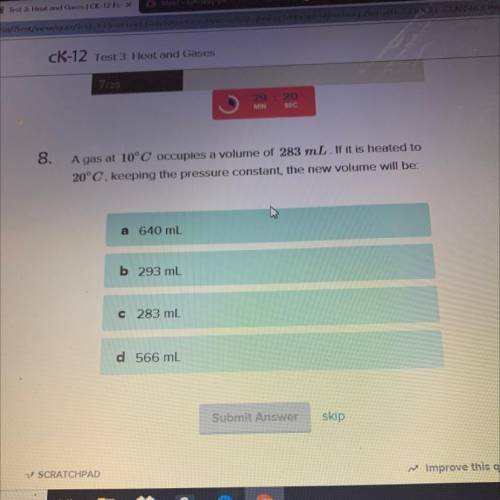
A gas at 10°C occupies a volume of 283 mL. If it is heated to
20°C, keeping the pressure constant,...
Questions in other subjects:

Mathematics, 16.04.2020 19:52



Mathematics, 16.04.2020 19:52

Social Studies, 16.04.2020 19:53


Mathematics, 16.04.2020 19:53



Mathematics, 16.04.2020 19:53