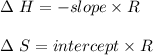A. Briefly explain how a loss of water by evaporation would affect the initial calculation of the solubility of your salt. B. Would this initial evaporation affect the calculated solubility of your salt at each subsequent experimental saturation temperature, or just the initial temperature? Explain.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, hjamya17
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2


Chemistry, 22.06.2019 21:00, lucyamine0
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1
You know the right answer?
A. Briefly explain how a loss of water by evaporation would affect the initial calculation of the so...
Questions in other subjects:

Mathematics, 10.09.2020 16:01

Mathematics, 10.09.2020 16:01

Mathematics, 10.09.2020 16:01

English, 10.09.2020 16:01

Mathematics, 10.09.2020 16:01

Mathematics, 10.09.2020 16:01

Mathematics, 10.09.2020 16:01

Mathematics, 10.09.2020 16:01

Mathematics, 10.09.2020 16:01

Mathematics, 10.09.2020 16:01

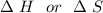 are calculated by
are calculated by 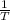 v/s lnKsp
v/s lnKsp