Iron(ii) sulfide reacts with hydrochloric acid according to the reaction:
fes(s)+2hcl(aq)→fe...

Chemistry, 17.10.2019 03:30 amberchule9681
Iron(ii) sulfide reacts with hydrochloric acid according to the reaction:
fes(s)+2hcl(aq)→fecl2(s)+h2s(g)
a reaction mixture initially contains 0.240 mol fes and 0.646 mol hcl.
what amount (in moles) of the excess reactant is left?

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
Questions in other subjects:

Mathematics, 22.01.2021 23:50



Mathematics, 22.01.2021 23:50

Arts, 22.01.2021 23:50

Social Studies, 22.01.2021 23:50




Mathematics, 22.01.2021 23:50

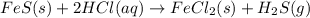
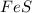 react with 2 mole of
react with 2 mole of 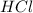
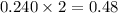 moles of
moles of 

