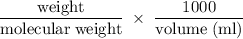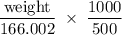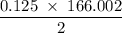Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, muncyemily
If a 60-g object has a volume of 30 cm3, what is its density? 2 g/cm3 0.5 cm3/g 1800 g * cm3 none of the above
Answers: 3

Chemistry, 21.06.2019 22:20, Brooke7644
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 21.06.2019 22:50, ajaydonlee
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
2. Calculate the number of grams of potassium iodide (KI), needed to prepare 500.0 mL of a
0.125 M...
Questions in other subjects:

History, 25.08.2020 14:01



Mathematics, 25.08.2020 14:01

Chemistry, 25.08.2020 14:01

English, 25.08.2020 14:01

Chemistry, 25.08.2020 14:01


Mathematics, 25.08.2020 14:01

Mathematics, 25.08.2020 14:01