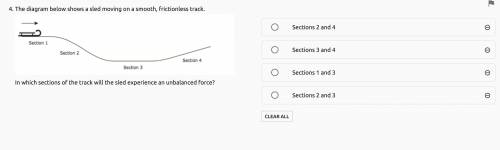
Can someone answer this question? It's not that long
...

Chemistry, 19.11.2020 01:00 goreimani9
Can someone answer this question? It's not that long

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:50, kelli151
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 12:00, luffybunny
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 13.09.2020 22:01

Geography, 13.09.2020 22:01

Mathematics, 13.09.2020 22:01

Mathematics, 13.09.2020 22:01

Mathematics, 13.09.2020 22:01

Mathematics, 13.09.2020 22:01

Mathematics, 13.09.2020 22:01

Mathematics, 13.09.2020 22:01

Mathematics, 13.09.2020 22:01

Mathematics, 13.09.2020 22:01



