
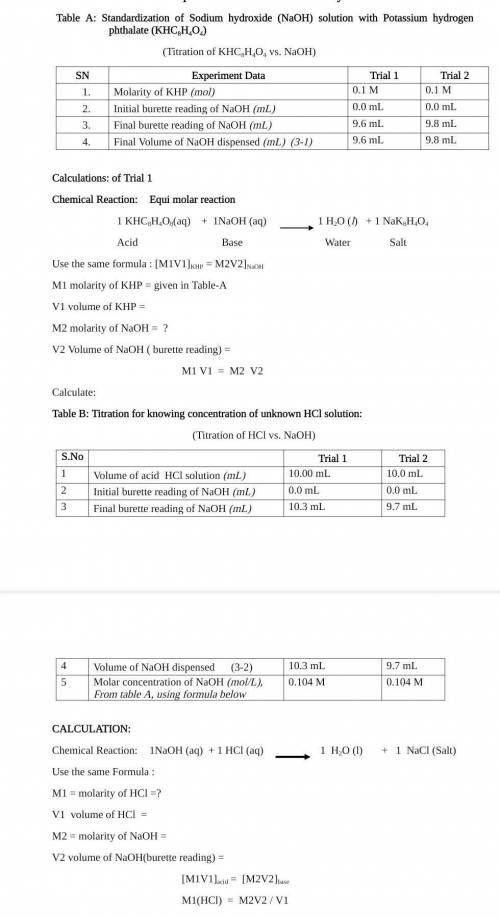
Standardization of Sodium hydroxide (NaOH) solution with Potassium
hydrogen phthalate (KHC2H404)
Table B: Titration for knowing concentration of unknown HCI solution:
(Titration of HCl vs. NaOH)
(Titration of KHCSH:04 vs. NaOH)
S. No
Trial 1
SN
1.
2.
3.
4.
Experiment Data
Molarity of KHP (nol)
Initial burette reading of NaOH (MI)
Fmal burette reading of NaOH (ml)
Final Volume of NaOH dispensed (m) (3-1)
Trial
01M
Q0 ml
9.6 TL
9.0
Trial 2
QUIM
0.0 mL
9.8 ml
9.5 mL
Volume of rid HCl solution (m)
10.00 mL
Intial burente reading of NaOH (m2
0.0 ml
Final burette reading of NaOH (ml)
103 m.
Volume of NaOH dispenyed (3-2)
10.3 ml
Mola concentribon of NaOH (mol). From table A, using 02104 M
Fortula balow
Trial 2
10 0 TL
0.0 ml
9.7 ml
9.1 ml
0.104 M
Calculations: of Trial 1
CALCULATION:
Chemical Reaction:
Equi molar reaction
1 H2O (1)
+ 1 NaCl
1 KHC8H4O3(aq) + 2NaOH(aq)
Chemical Reaction1NaOH (OCT + 1 HCI (aq)
(Salt)
1 H2O(l) +1 NK H404
Acid
Base
Water
Salt
Use the same Formula:
Use the same formula : MIV1]KHP = M2V2]NaOH
MI = molarity of HCI=?
M1 molarity of KHP=0.1 M
Vi volume of HCl =
V1 volume of KHP =
M2 =molarity of NaOH =
M2 molarity of NaOH = ?
V2 volume of NaOH(burette reading) =
V2 Volume of NaOH (burette reading) =
MIV1]acid = [MV2]b.
M1 V1 = M2 V2
M1(HCI) = M2V2/V1
Calculate:
Screens 1-2 of 2

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, foreignking02
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3


Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 22:30, angelagonzalesownus1
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
Standardization of Sodium hydroxide (NaOH) solution with Potassium
hydrogen phthalate (KHC2H404)
Questions in other subjects:



English, 22.09.2019 03:50


Mathematics, 22.09.2019 03:50

Geography, 22.09.2019 03:50

History, 22.09.2019 03:50

English, 22.09.2019 03:50

Mathematics, 22.09.2019 03:50



