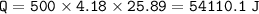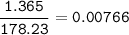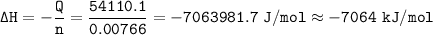When 1.365 g of anthracene, C14H10, is combusted in a bomb calorimeter that has a water jacket containing 500.0 g of water, the temperature of the water increases by 25.89°C. Assuming that the specific heat of water is 4.18 J/(g ∙°C), and that the heat absorption by the calorimeter is negligible, estimate the enthalpy of combustion per mole of anthracene.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:10, gungamer720
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
When 1.365 g of anthracene, C14H10, is combusted in a bomb calorimeter that has a water jacket conta...
Questions in other subjects:





Mathematics, 19.05.2021 20:50

Mathematics, 19.05.2021 20:50

Geography, 19.05.2021 20:50


Mathematics, 19.05.2021 20:50

Mathematics, 19.05.2021 20:50