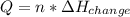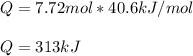Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, ctyrector
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1


You know the right answer?
Calculate the quantity of energy required to change 7.72 mol of liquid water to steam at 100oC. The...
Questions in other subjects:







Mathematics, 07.09.2020 02:01

Law, 07.09.2020 02:01