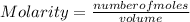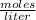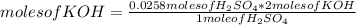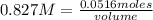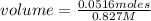Chemistry, 18.11.2020 17:10 ahmedeldyame
In a titration of 35.00 mL of 0.737 M H2SO4, mL of a 0.827 M KOH solution is required for neutralization.
A) 35.0
B) 1.12
C) 25.8
D) 62.4
E) 39.3

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:30, leslyrivera11
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

You know the right answer?
In a titration of 35.00 mL of 0.737 M H2SO4, mL of a 0.827 M KOH solution is required for neutraliz...
Questions in other subjects:


Mathematics, 10.06.2021 23:50



Mathematics, 10.06.2021 23:50

Computers and Technology, 10.06.2021 23:50

Mathematics, 10.06.2021 23:50

Mathematics, 10.06.2021 23:50