
Chemistry, 18.11.2020 07:50 swaggernas
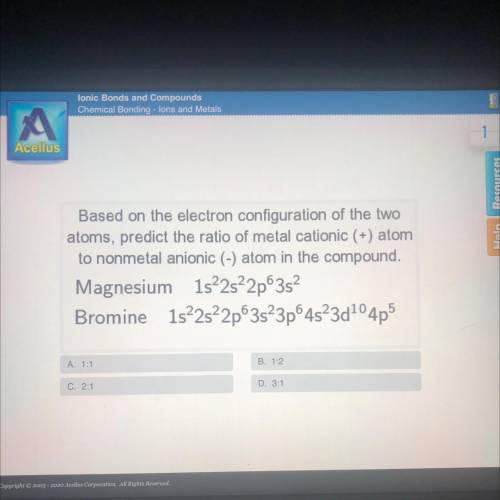
Based on the electron configuration of the two
atoms, predict the ratio of metal cationic (+) atom
to nonmetal anionic (-) atom in the compound.
Magnesium 1s22s22p3s
Bromine 1522s22p63s23p64523d104p5
A. 1:1
B. 1:2
C. 2:1
D. 3:1

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 00:30, evelynalper08
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
Based on the electron configuration of the two
atoms, predict the ratio of metal cationic (+) atom<...
Questions in other subjects:

Mathematics, 10.11.2019 04:31

Computers and Technology, 10.11.2019 04:31


English, 10.11.2019 04:31


English, 10.11.2019 04:31



Mathematics, 10.11.2019 04:31



