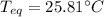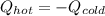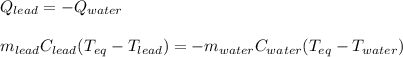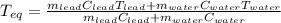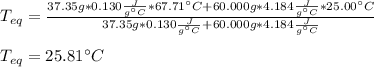Chemistry, 18.11.2020 02:40 diamondk2019
A chemist heats 37.35 g of lead to 67.71 °C , then places the metal sample in the cup of water shown in the interactive. Calculate the final temperature of the water. The specific heat of lead is 0.130 J/g⋅°C and the specific heat of water is 4.184 J/g⋅°C .
mass of water: 60.000g
initial temperature of water: 25.00 C

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, aubreykenzie686
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 18:00, kamjay2006
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1


Chemistry, 22.06.2019 21:30, djdjdjdbdbjx
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
A chemist heats 37.35 g of lead to 67.71 °C , then places the metal sample in the cup of water shown...
Questions in other subjects:

Biology, 13.01.2021 20:30


Mathematics, 13.01.2021 20:30



Mathematics, 13.01.2021 20:30

Mathematics, 13.01.2021 20:30


History, 13.01.2021 20:30