
Chemistry, 18.11.2020 01:50 khenalilovespandas
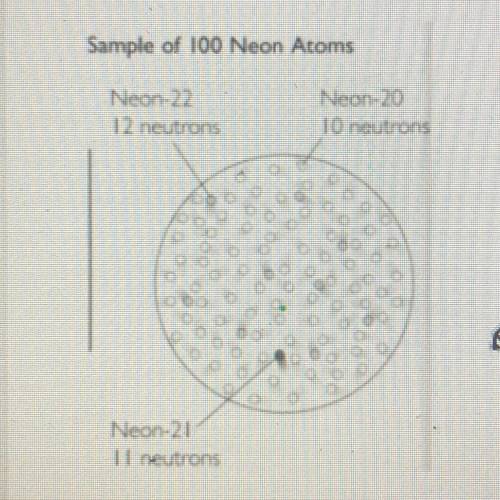
Paul was looking at the sample of Neon isotopes below.
He calculated average atomic mass by doing the following: 20 + 21 + 22 = 63 / 3 = 21 amu
Paul is told that his answer is incorrect but doesn't know why. Explain what's wrong with his calculation & answer

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, soliseric879
Dwayne filled a small balloon with air at 298.5 k. he put the balloon into a bucket of water, and the water level in the bucket increased by 0.54 liter. if dwayne puts the balloon into a bucket of ice water at 273.15 k and waits for the air inside the balloon come to the same temperature, what will the volume of the balloon be? assume the pressure inside the balloon doesn’t change. type the correct answer in the box. express your answer to the correct number of significant figures. the volume of the balloon at 273.15 k is liters.
Answers: 2

Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

You know the right answer?
Paul was looking at the sample of Neon isotopes below.
He calculated average atomic mass by doing t...
Questions in other subjects:


Mathematics, 01.04.2021 17:20




Mathematics, 01.04.2021 17:20



Mathematics, 01.04.2021 17:20




