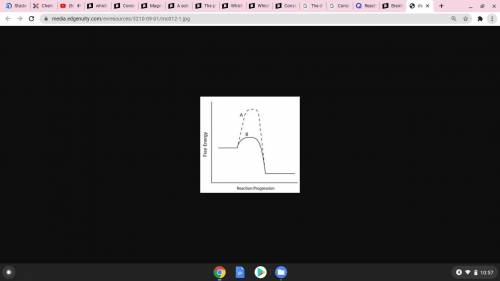
Consider the energy diagram below.
Which line indicates a higher reaction rate?
A because it...


Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 23:20, svaskeacevilles5477
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2
You know the right answer?
Questions in other subjects:

Biology, 20.10.2020 05:01







Mathematics, 20.10.2020 05:01


English, 20.10.2020 05:01