
Chemistry, 17.11.2020 17:30 rodriguezbrian050702
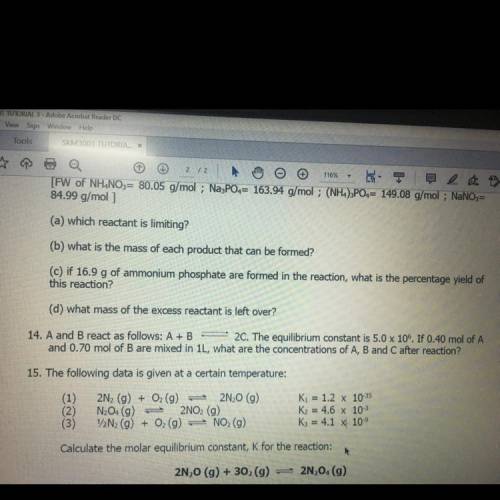
14. A and B react as follows: A+B 2C. The equilibrium constant is 5.0 x 106. If 0.40 mol of A and 0.70 mol of B are mixed in 1L, what are the concentrations of A, B and C after reaction?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, khalaflaf2684
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

You know the right answer?
14. A and B react as follows: A+B 2C. The equilibrium constant is 5.0 x 106. If 0.40 mol of A
and 0...
Questions in other subjects:


Mathematics, 04.05.2021 08:50

Mathematics, 04.05.2021 08:50

Mathematics, 04.05.2021 08:50

Mathematics, 04.05.2021 08:50



Mathematics, 04.05.2021 08:50

Mathematics, 04.05.2021 08:50



