PLEASE HELP WITH EXPLINATION ;(
according to the reaction Zn + 2HCl ⇒ZnCl2 +H2
what is the pr...


Answers: 2


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 08:30, lowkstahyna
What percentage of energy used in the u. s is produced from fossil fuels
Answers: 2
You know the right answer?
Questions in other subjects:

Mathematics, 26.01.2020 00:31




History, 26.01.2020 00:31



Chemistry, 26.01.2020 00:31

Biology, 26.01.2020 00:31

Mathematics, 26.01.2020 00:31

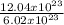 = 2moles of H₂
= 2moles of H₂

