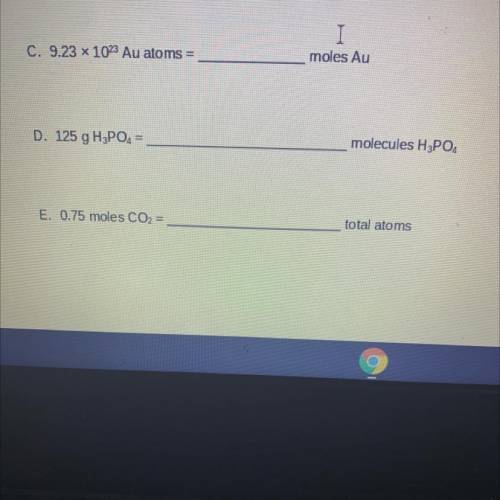
C. 9.23 x 1023 Au atoms =
moles Au
D. 125 g H3PO4 =
molecules H3PO4
E. 0.75 moles...

Chemistry, 17.11.2020 08:20 kimberlyblanco14
C. 9.23 x 1023 Au atoms =
moles Au
D. 125 g H3PO4 =
molecules H3PO4
E. 0.75 moles CO2 =
total atoms
help pls due in 5 min

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:30, ricardotavarez6
How does a large body of water, such as the ocean, influence climate?
Answers: 1
You know the right answer?
Questions in other subjects:





Health, 26.01.2021 21:50



Chemistry, 26.01.2021 21:50

English, 26.01.2021 21:50



