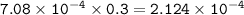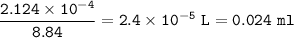Chemistry, 17.11.2020 06:30 jrfranckowiak
What volume (in mL) of 8.84 M HBr would be required to make 300.0 mL of a solution with a pH of 3.15?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, jadepotts3965
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 21.06.2019 23:00, carter1809
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 00:30, jamesnaquan132
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 04:20, lindseysmith9522
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1
You know the right answer?
What volume (in mL) of 8.84 M HBr would be required to make 300.0 mL of a solution with a pH of 3.15...
Questions in other subjects:

History, 09.03.2021 03:50

Mathematics, 09.03.2021 03:50






Physics, 09.03.2021 03:50

English, 09.03.2021 03:50

Mathematics, 09.03.2021 03:50

![\tt [H^+]=10^{-3.15}=7.08\times 10^{-4}](/tpl/images/0903/9462/83b78.png)