
Chemistry, 16.11.2020 20:20 meganwintergirl
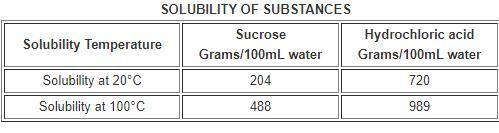
The table below compares the solubility of two substances in 100 milliliters (mL) of water.
a. The solubility of sucrose increases if more is dissolved.
b. The solubility of sucrose decreases as temperature increases
c. The solubility of hydrochloric acid increases if more is dissolved.
d. The solubility of hydrochloric acid increases as temperature increases.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:10, tishfaco5000
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 10:00, zionlopez543
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1


Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
The table below compares the solubility of two substances in 100 milliliters (mL) of water.
a. The...
Questions in other subjects:

History, 01.01.2020 07:31

Mathematics, 01.01.2020 07:31



Chemistry, 01.01.2020 07:31

Health, 01.01.2020 07:31







