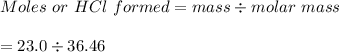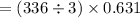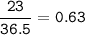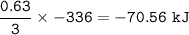Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 23.06.2019 01:00, breemills9552
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1
You know the right answer?
The value of ΔH° for the reaction below is -336 kJ. Calculate the heat (kJ) released to the surround...
Questions in other subjects:




History, 04.11.2020 14:00

History, 04.11.2020 14:00


Social Studies, 04.11.2020 14:00


English, 04.11.2020 14:00