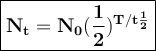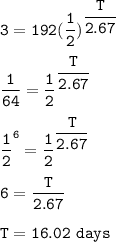Chemistry, 15.11.2020 23:10 elizabethxoxo3271
Yttrium-90, Y90 , is a radioactive isotope used in the treatment of liver cancer. The half‑life of Y90 is 2.67 days. If a dose with an activity of 192 μCi is given to a patient, how many days will it take for the activity of Y90 in the patient to reach 3.00 μCi?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, kitykay2776
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1


Chemistry, 23.06.2019 01:30, ayoismeisalex
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1

Chemistry, 23.06.2019 02:00, bagofmud8339
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
You know the right answer?
Yttrium-90, Y90 , is a radioactive isotope used in the treatment of liver cancer. The half‑life of Y...
Questions in other subjects:

Mathematics, 25.01.2021 02:20




Mathematics, 25.01.2021 02:20


Mathematics, 25.01.2021 02:20

Mathematics, 25.01.2021 02:20

Mathematics, 25.01.2021 02:20

Spanish, 25.01.2021 02:20