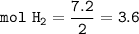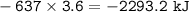Chemistry, 15.11.2020 20:40 calistaallen1734
The thermodynamic information for the following reaction is as follows:
HNO3 (g) + H2 (g) → NH3 (g) + H2O (g) △H = −637 kJ
1) Balance the chemical reaction.
2) Identify this reaction as endothermic or exothermic.
3) Calculate how much heat is released when 7.20 g of H2 reacts in this situation.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, youngchapo813p8d9u1
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 11:40, tatemelliott
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3


Chemistry, 23.06.2019 02:30, micahwilkerson9495
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1
You know the right answer?
The thermodynamic information for the following reaction is as follows:
HNO3 (g) + H2 (g) → NH3 (g)...
Questions in other subjects:

Spanish, 09.02.2021 18:40


Mathematics, 09.02.2021 18:40





Mathematics, 09.02.2021 18:40

Mathematics, 09.02.2021 18:40

History, 09.02.2021 18:40