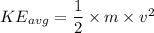The graph above shows the distribution of molecular speeds for four different gases at the same temperature. What property of the different gases can be correctly ranked using information from the graph, and why?
(Graph attached) I WILL MARK BRAINLIEST
A.) The densities of the gases, because as the density of a gas increases, the average speed of its molecules decreases.
B.) The pressures of the gases, because the pressure exerted by a gas depends on the average speed with which its molecules are moving.
C.) The volumes of the gases, because at a fixed temperature the volume of a gas can be calculated using the equation PV=nRT.
D.) The molecular masses of the gases, because the gas molecules have the same average kinetic energy and mass can be calculated using the equation KEavg=12mv2

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, marcusajns
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 03:10, emilyplays474
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2
You know the right answer?
The graph above shows the distribution of molecular speeds for four different gases at the same temp...
Questions in other subjects:


Mathematics, 29.12.2019 19:31

Biology, 29.12.2019 19:31





Social Studies, 29.12.2019 19:31

Mathematics, 29.12.2019 19:31

History, 29.12.2019 19:31