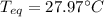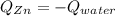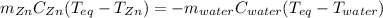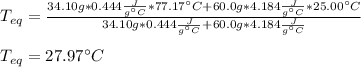Chemistry, 15.11.2020 01:00 george6871
A chemist heats 34.10 g of nickel to 77.17 °C, then places the metal sample in the cup of water shown in the interactive.
Calculate the final temperature of the water. The specific heat of nickel is 0.444 J/g °C and the specific heat of water is
4.184 J/g °C.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 07:00, ashleyrturner08
Explain what happened when the storm surges from hurricanes reached the gulf coast
Answers: 1
You know the right answer?
A chemist heats 34.10 g of nickel to 77.17 °C, then places the metal sample in the cup of water show...
Questions in other subjects:


Computers and Technology, 22.01.2021 08:30


History, 22.01.2021 08:30



Mathematics, 22.01.2021 08:30

Mathematics, 22.01.2021 08:30

Mathematics, 22.01.2021 08:30

Mathematics, 22.01.2021 08:30