

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 11:20, mjwenz8018
Ajar is tightly sealed at 22°c and 772 torr what is the pressure inside a jar after its been heated to 178°c
Answers: 1

Chemistry, 23.06.2019 12:30, ella3714
17) large amounts of very important metal titanium are made by reacting magnesium metal with titanium tetrachloride. titanium metal and magnesium chloride are produced. a) write the balanced equation for this reaction. b) how many kilograms of magnesium are required to produce 1.00 kilograms of titanium? ( show work, .)
Answers: 1


Chemistry, 23.06.2019 13:00, Crxymia
How long could you survive without electricity? what parts of your life would be affected by loss of electricity? should you prepare for an electricity outage, and if so, how would you prepare? what backup system could your family or community install to generate limited amounts of electricity during an outage? how does this system create an electric force field and generate electric current?
Answers: 2
You know the right answer?
How many moles of hydrogen are needed to produce 8 moles of methane CH4? C + H2-> CH4...
Questions in other subjects:

Health, 16.12.2020 07:20

Mathematics, 16.12.2020 07:20

Mathematics, 16.12.2020 07:20

Social Studies, 16.12.2020 07:20

Biology, 16.12.2020 07:20

Mathematics, 16.12.2020 07:20

History, 16.12.2020 07:20


Social Studies, 16.12.2020 07:20

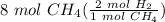 = 16 mol H₂
= 16 mol H₂

