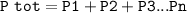Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:30, elizabethprasad2
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
A mixture of two gases with a total pressure of 2.00 atm contains 0.70 atm of Gas A. What is the par...
Questions in other subjects:


Chemistry, 12.05.2021 18:50



Mathematics, 12.05.2021 18:50

English, 12.05.2021 18:50


Mathematics, 12.05.2021 18:50

Mathematics, 12.05.2021 18:50

Mathematics, 12.05.2021 18:50