
7.
A 27.8 L balloon is filled with 7.52g of gas X and placed next to a 27.8 L balloon filled with helium.
Given that Helium has a density of 0.179g/L, which statement correctly explains the movement of the balloons
with valid justification?
a) The balloon filled with helium will float higher because helium is less dense than gas X
b) The balloon filled with gas X will float higher because gas X is less dense than helium
c) Both balloons will float to the same level because their densities are the same
d) Both balloons will float to the same level because their volumes are the same
e) There is not enough information to determine the movement of the balloons

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, jojomgarcia01
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 15:00, hockeykid7583
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 16:40, westball101
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
7.
A 27.8 L balloon is filled with 7.52g of gas X and placed next to a 27.8 L balloon filled with h...
Questions in other subjects:





Physics, 21.09.2019 03:10



Mathematics, 21.09.2019 03:10


Biology, 21.09.2019 03:10

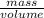
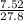 = 0.271g/L
= 0.271g/L 

