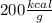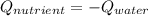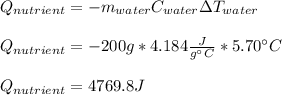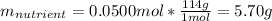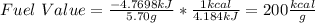Chemistry, 11.11.2020 23:20 khikhi1705
7.46 - A 0.0500-mol sample of a nutrient substance is burned in a bomb calorimeter containing 2.00 x 10g H20. If the formula weight of this nutrient substance is 114 g/mol, what is the fuel value (in nutritional Cal) if the temperature of the water increased 5.70C?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, scottbrandon653
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 01:00, chrisxxxrv24
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

You know the right answer?
7.46 - A 0.0500-mol sample of a nutrient substance is burned in a bomb calorimeter containing 2.00 x...
Questions in other subjects:




Health, 25.09.2020 05:01



Mathematics, 25.09.2020 05:01

Biology, 25.09.2020 05:01


Mathematics, 25.09.2020 05:01