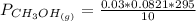Please answer I have a finals and I need help with a question similar!!! Methanol can be synthesized in the gas phase by the reaction of gas phase carbon monoxide with gas phase hydrogen. A 10.0 L reaction flask contains carbon monoxide gas at 0.461 bar and 22.0 °C. 345 mL of hydrogen gas at 7.12 bar and 271 K is introduced. Assume the reaction goes to completion (100% yield). What are the partial pressures of each gas at the end of the reaction, once the temperature has returned to22.0 °C?. units in bar please

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, graciewyatt6833
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 19:00, elizabethajih99
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
You know the right answer?
Please answer I have a finals and I need help with a question similar!!! Methanol can be synthesized...
Questions in other subjects:



Mathematics, 14.07.2019 09:30







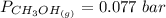
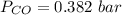
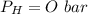
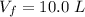
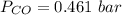
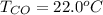
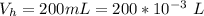
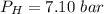
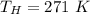
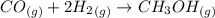
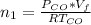
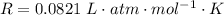
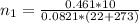
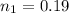
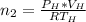

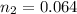
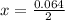
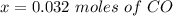

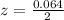
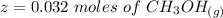
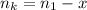
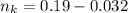
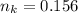
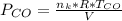
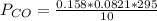
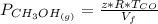
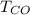 is used because it is given the question that the temperature returned to 22.0 degrees C
is used because it is given the question that the temperature returned to 22.0 degrees C