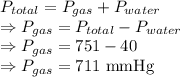Chemistry, 11.11.2020 09:00 Irishstoner5608
A laboratory hydrogen generator collects the gas produced by bubbling it through water. The total pressure of the gas collected is 751 mmHg. The temperature is 34 °C, at which water vapor pressure is 40.0 mmHg. Calculate the partial pressure of the hydrogen.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, hannahkelly3618
How many moles are equivalent to 55.5g of nano3
Answers: 1


You know the right answer?
A laboratory hydrogen generator collects the gas produced by bubbling it through water. The total pr...
Questions in other subjects:


Mathematics, 07.01.2021 22:00


Mathematics, 07.01.2021 22:00

Mathematics, 07.01.2021 22:00


History, 07.01.2021 22:00

Health, 07.01.2021 22:00


Mathematics, 07.01.2021 22:00

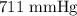
 = Total pressure = 751 mmHg
= Total pressure = 751 mmHg = Vapor pressure = 40 mmHg
= Vapor pressure = 40 mmHg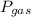 = Pressure of gas
= Pressure of gas