
Part 2: Identifying Substances with the Flame Test
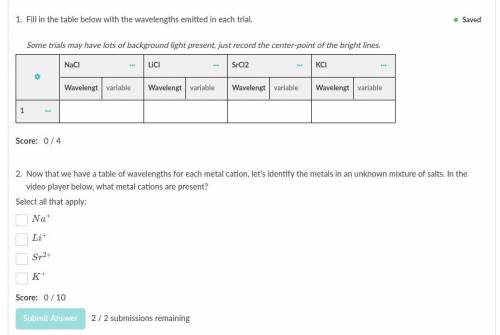
We've figured out what part of the salt causes the flame to change color, so now let's measure the wavelengths created with four metals.
Use the ruler under the "tools" icon in the upper right of the video player to measure the wavelengths of light released by each compound.

Answers: 2


Other questions on the subject: Chemistry



You know the right answer?
Part 2: Identifying Substances with the Flame Test
We've figured out what part of the salt causes t...
Questions in other subjects:


Mathematics, 26.09.2019 10:00

Social Studies, 26.09.2019 10:00



Mathematics, 26.09.2019 10:00


Mathematics, 26.09.2019 10:10





