Chemistry, 10.11.2020 20:10 aydenmasonc
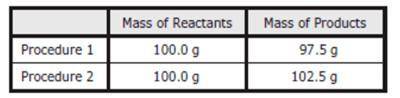
As part of an investigation, students combined substances in a beaker to observe chemical reactions. They performed two procedures. They measured the mass of each substance before and after each reaction. The table shows their observations. Assuming the students did not make any careless errors, what likely explains these changes in mass?
Question 3 options:
Procedure 1: All the reactants were liquids that evaporated. Procedure 2: A gas was formed as one product, and it escaped into the air.
Procedure 1: One of the reactants was converted to thermal energy. Procedure 2: All the products were liquids.
Procedure 1: The reactants were liquids with different densities. Procedure 2: The reactants were combined into only one product.
Procedure 1: One of the products was a gas that escaped into the air. Procedure 2: A gas from the air reacted with one of the other reactants.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, lpssprinklezlps
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3



Chemistry, 23.06.2019 01:00, dawnparker71
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3
You know the right answer?
As part of an investigation, students combined substances in a beaker to observe chemical reactions....
Questions in other subjects:


Arts, 15.01.2021 19:10

English, 15.01.2021 19:10







Business, 15.01.2021 19:10