
Chemistry, 10.11.2020 09:20 Josephjenkins620
The molecular mass of air, at standard pressure and temperature, is approximately 28.97 g/mol.
Calculate the mass of 3.33 moles of air.
First, complete the unit conversion using dimensional analysis:
A • B/C
A: 3.33 mil air
B: 28.87 g air
C: 1 mol air

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mayamabjishovrvq9
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 14:30, Tooey2331
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 14:30, amylumey2005
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
The molecular mass of air, at standard pressure and temperature, is approximately 28.97 g/mol.
Calc...
Questions in other subjects:


Mathematics, 23.09.2019 03:20



History, 23.09.2019 03:20


Biology, 23.09.2019 03:20



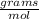 * 3.33 moles
* 3.33 moles

