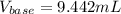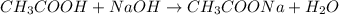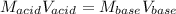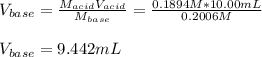Chemistry, 09.11.2020 16:50 brooke7768
Given that 10.00mL of 0.1894M CH3COOH was titrated with 0.2006M NaOH in this experiment, calculate the volume, in mL, of NaOH required

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 17:00, brandiwingard
What is the mass of phosphorous in a 51-kg person
Answers: 1

Chemistry, 23.06.2019 08:00, IntellTanito
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 2

Chemistry, 23.06.2019 10:30, 7thaohstudent
What is the difference between skimming and absorbing methods of the oil removal
Answers: 2
You know the right answer?
Given that 10.00mL of 0.1894M CH3COOH was titrated with 0.2006M NaOH in this experiment, calculate t...
Questions in other subjects:



English, 23.03.2020 20:30

Biology, 23.03.2020 20:30

Mathematics, 23.03.2020 20:30



Physics, 23.03.2020 20:30