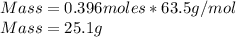Q2. A 0.696 mol sample of Cu is added to 146 mL of 5.5 M HNO3. Assuming the following
reaction is only one that occurs;
Cus) + HNO3(aq) → Cu(NO3)2(aq) + H2O() + NO()
Will the Cu react completely? What is the limiting reagent and what is the remaining compound
in mass?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2



Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
You know the right answer?
Q2. A 0.696 mol sample of Cu is added to 146 mL of 5.5 M HNO3. Assuming the following
reaction is o...
Questions in other subjects:


Mathematics, 08.04.2021 16:10








Mathematics, 08.04.2021 16:10