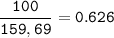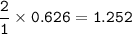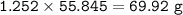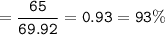Chemistry, 08.11.2020 16:40 athenajames1221
Iron is extracted from iron oxide in the Blast Furnace: Fe 2 O 3 + 3 CO → 2 Fe + 3 CO 2
a) Calculate the maximum theoretical mass of iron that can be made from 100g
of iron oxide.
b) In the reaction, only 65 g of iron was made. Calculate the percentage yield.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, mosthatedpicky1
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2


Chemistry, 23.06.2019 01:00, kaykardash
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2

Chemistry, 23.06.2019 01:30, Michael845313
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1
You know the right answer?
Iron is extracted from iron oxide in the Blast Furnace: Fe 2 O 3 + 3 CO → 2 Fe + 3 CO 2
a) Calculat...
Questions in other subjects:

Computers and Technology, 23.06.2021 21:00



Mathematics, 23.06.2021 21:00

Mathematics, 23.06.2021 21:00


Mathematics, 23.06.2021 21:00

History, 23.06.2021 21:00


Mathematics, 23.06.2021 21:00