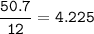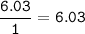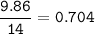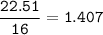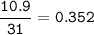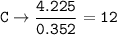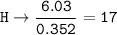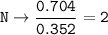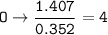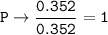Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:50, karlyisaunicorn
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2


Chemistry, 22.06.2019 13:30, yasiroarafat12
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
5. Psilocybin is made of C 50.70%, H 6.03%, N 9.86%, 22.51%, P 10.90%. a. Find the empirical formula...
Questions in other subjects:

Geography, 20.05.2021 14:00



Chemistry, 20.05.2021 14:00

Mathematics, 20.05.2021 14:00




Mathematics, 20.05.2021 14:00