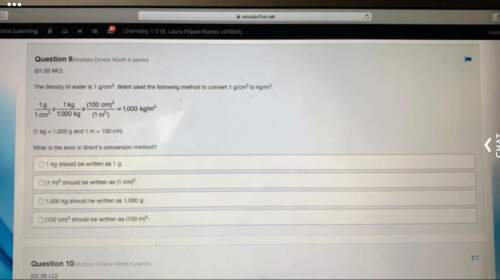
The forensic technician at a crime scene has just prepared a luminol stock solution by adding 15.0 g of luminol into a total volume of 75.0 mL of H2O. What is the molarity of the stock solution of luminol?(1.13M)
Before investigating the scene, the technician must dilute the luminol solution to a concentration of 3.00×10−2 M . The diluted solution is then placed in a spray bottle for application on the desired surfaces.
How many moles of luminol are present in 2.00 L of the diluted spray?

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:10, jojomgarcia01
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 23.06.2019 04:00, izzyp619
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
The forensic technician at a crime scene has just prepared a luminol stock solution by adding 15.0 g...
Questions in other subjects:

Spanish, 12.05.2021 05:10

Mathematics, 12.05.2021 05:10





Mathematics, 12.05.2021 05:10



Mathematics, 12.05.2021 05:10