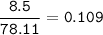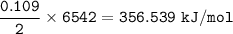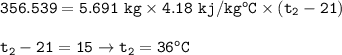Chemistry, 07.11.2020 05:40 joThompson
If 8.500 g CH is burned and the heat produced from the burning is added to 5691 g of water at 21 °C, what is the final temperature of the water?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, cicimarie2018
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1



Chemistry, 22.06.2019 21:00, rhondafits9000
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
If 8.500 g CH is burned and the heat produced from the burning is added to 5691 g of water at 21 °C,...
Questions in other subjects:

History, 28.06.2019 16:00


Mathematics, 28.06.2019 16:00

History, 28.06.2019 16:00



Mathematics, 28.06.2019 16:00

Mathematics, 28.06.2019 16:00


Mathematics, 28.06.2019 16:00