
Chemistry, 07.11.2020 01:00 chaycebell6662
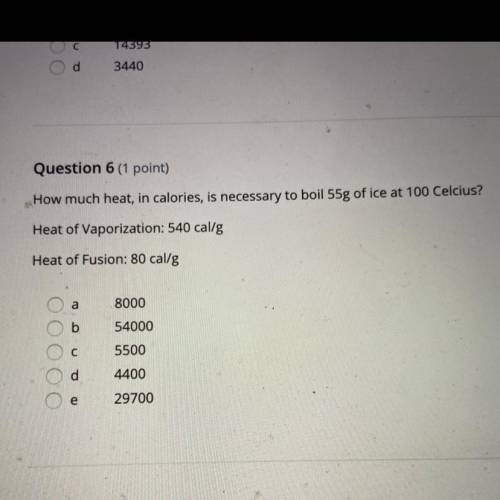
Question 6 (1 point)
How much heat, in calories, is necessary to boil 55g of ice at 100 Celcius?
Heat of Vaporization: 540 cal/g
Heat of Fusion: 80 cal/g
a
OO
С
8000
54000
5500
4400
29700
OC
e

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 23.06.2019 00:10, graceception
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 03:00, jaidencoolman2866
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
Question 6 (1 point)
How much heat, in calories, is necessary to boil 55g of ice at 100 Celcius?
Questions in other subjects:


Mathematics, 07.05.2020 00:12


Mathematics, 07.05.2020 00:12

Mathematics, 07.05.2020 00:12


English, 07.05.2020 00:12

Social Studies, 07.05.2020 00:12




