
Chemistry, 07.11.2020 01:00 katiekern5207
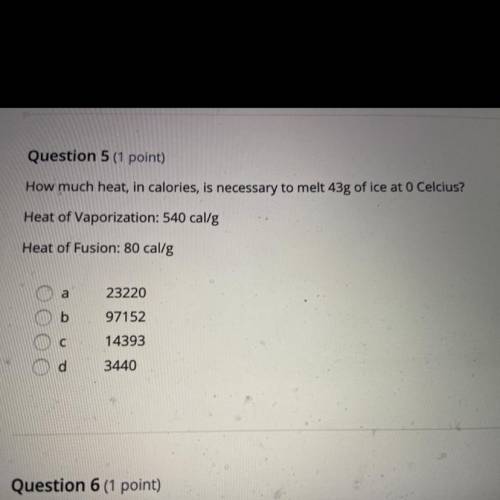
Question 5 (1 point)
How much heat, in calories, is necessary to melt 43g of ice at 0 Celcius?
Heat of Vaporization: 540 cal/g
Heat of Fusion: 80 cal/g
a
b
23220
97152
14393
с
d
3440

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 09:00, ashhull2002
Need ! assume that the variables x and y are directly related. if k = 8, what is the value for each of the following points? be sure and record your data to be used in the following problem. x y k 0.
Answers: 2

Chemistry, 23.06.2019 13:00, journeyburks07
The gram molecular mass or co2 is the same as the gram molecular mass of
Answers: 2
You know the right answer?
Question 5 (1 point)
How much heat, in calories, is necessary to melt 43g of ice at 0 Celcius?
Questions in other subjects:



Mathematics, 25.05.2021 20:10

Mathematics, 25.05.2021 20:10



Chemistry, 25.05.2021 20:10

Mathematics, 25.05.2021 20:10





