
Chemistry, 26.01.2020 01:31 havanaoohnana
To form an oxygen molecule (02), two oxygen atoms share two pairs of electrons. what kind of bond is shown below by the electron dot diagram of 02? choose all that apply.
2 answers
1) covalent bond
2) ionic bond
3) single bond
4) double bond
5) triple bond
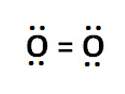

Answers: 3


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
To form an oxygen molecule (02), two oxygen atoms share two pairs of electrons. what kind of bond is...
Questions in other subjects:

History, 02.02.2020 19:50

Mathematics, 02.02.2020 19:50

Mathematics, 02.02.2020 19:50




Mathematics, 02.02.2020 19:50

Mathematics, 02.02.2020 19:50

Social Studies, 02.02.2020 19:50

Mathematics, 02.02.2020 19:51



