
Chemistry, 06.11.2020 23:10 kokilavani
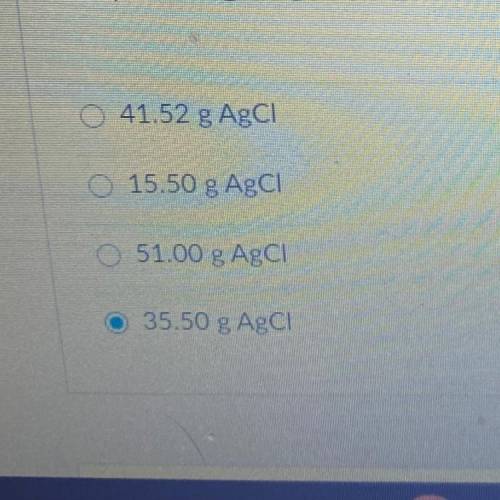
15.50 g of NH4Cl reacts with an excess of AgNO3. In the reaction 35.50 g
AgCl is produced. What is the percent yield of AgCl?
NH4Cl + AgNO3 --> AgCl + NH4NO3
69.61 % Yield AgCl
0 43.66 % Yield AgCl
85.50 % Yield AgCI
O 85.50 g AgCI

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, david838843
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
15.50 g of NH4Cl reacts with an excess of AgNO3. In the reaction 35.50 g
AgCl is produced. What is...
Questions in other subjects:

Mathematics, 04.06.2021 04:00

Mathematics, 04.06.2021 04:00


Mathematics, 04.06.2021 04:00

Biology, 04.06.2021 04:00


Chemistry, 04.06.2021 04:00


Mathematics, 04.06.2021 04:00



