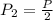Chemistry, 06.11.2020 22:50 davidswafforddd478
Boyles Law P1V1 = P2V2
An arrangement of helium balloons in the back of a delivery truck travels from a town at the base of a mountain up the mountain where the air pressure is half the amount. What do you expect to happen to the balloons?
A. The balloons will increase to twice their original volume.
B. The balloons will decease to one fourth their original size.
.C. The balloons will increase to four times their original size.
D. The balloons will decrease to half their original size.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 01:00, stefaniethibodeaux
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Boyles Law P1V1 = P2V2
An arrangement of helium balloons in the back of a delivery truck travels fr...
Questions in other subjects:

History, 22.10.2020 02:01



Mathematics, 22.10.2020 02:01

English, 22.10.2020 02:01

Physics, 22.10.2020 02:01

Chemistry, 22.10.2020 02:01

English, 22.10.2020 02:01


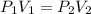
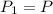 , initial volume be V, i.e.
, initial volume be V, i.e. 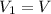 . The pressure is then halved, i.e.
. The pressure is then halved, i.e.