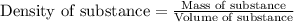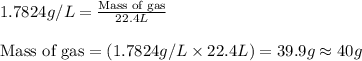Chemistry, 20.10.2019 21:30 unknown9263
The density of an elemental gas is 1.7824 g/l at stp. what is the molar mass of the element. what is the element?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 16:30, monithebtslover01
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 02:20, ayoismeisalex
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1
You know the right answer?
The density of an elemental gas is 1.7824 g/l at stp. what is the molar mass of the element. what is...
Questions in other subjects:


Mathematics, 29.01.2021 19:40



Physics, 29.01.2021 19:40


Mathematics, 29.01.2021 19:40