Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, brasherfamily14
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1

Chemistry, 22.06.2019 03:50, Pizzapegasus1
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 05:00, foreignking02
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3
You know the right answer?
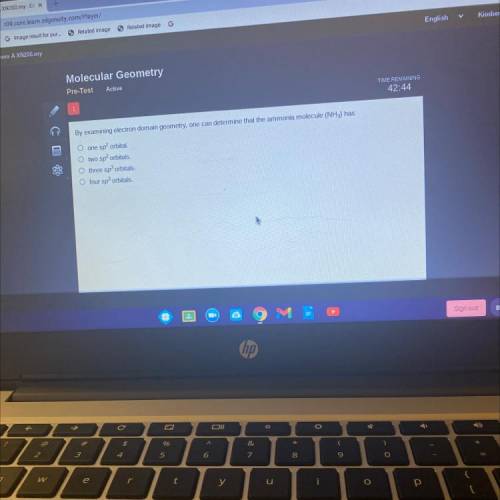
By examining electron domain geometry, one can determine that the ammonia molecule (NH3) has
one sp...
Questions in other subjects:


History, 05.05.2020 17:19

Mathematics, 05.05.2020 17:19





Biology, 05.05.2020 17:19

Mathematics, 05.05.2020 17:19