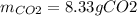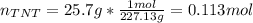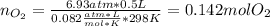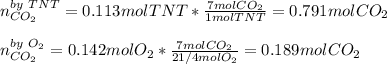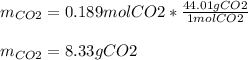Trinitrotoluene (TNT, C7H5N3O6) undergoes complete combustion according to the following balanced chemical equation:
C7H5N3O6(s)+214O2(g)→7CO2(g)+32N2(g )+52H2O(l)
If 25.7 g of TNT is combusted in a 0.500 L container filled with O2 at a pressure of 7.02 bar and a temperature of 298 K, calculate the maximum mass of CO2 that could be produced.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
You know the right answer?
Trinitrotoluene (TNT, C7H5N3O6) undergoes complete combustion according to the following balanced ch...
Questions in other subjects:

Mathematics, 09.04.2021 20:50






Mathematics, 09.04.2021 20:50